Abstract
Background: Ibrutinib is an orally-administered inhibitor of Bruton's tyrosine kinase. It is an effective agent in diffuse large B cell lymphoma (DLBCL), specifically of the Activated (ABC) type (Younes A et al, Lancet Oncol 2014). The combination of ibrutinib, bendamustine, and rituximab (IBR) was reported to be potent for B cell lymphomas, including DLBCL, in a phase I clinical trial (Maddocks et al, Blood 2015). We aimed to explore the efficacy of the IBR regimen in patients with relapsed & refractory (RR) aggressive B cell lymphoma (ABCL), who were either transplant ineligible or in relapse post autologous stem cell transplantation (ASCT). We report here, the interim analysis of a phase II clinical trial.
Methods: Patients with RR ABCL of all subtypes as well as transformed low-grade lymphoma and grade 3B follicular lymphoma were eligible. Patients with CNS lymphoma, inadequate bone marrow function or previous exposure to bendamustine or ibrutinib were excluded. All diagnostic biopsies were centrally reviewed or repeated. PET scans were interpreted according to the Lugano criteria 2014. Rituximab and bendamustine were given for a six 28 days cycles in their standard dose (rituximab 375 mg/m2 in day 1 and bendamustine 90/70 mg/m2 day 1 and 2). Ibrutinib 560 mg orally, was administered continuously from day 1. PET scans were repeated after 3 and 6 cycles of therapy and then every 16 weeks up to 3 years. Patients that achieved a response after 3 cycles and who were eligible, could undergo either allogeneic or autologous stem cell transplantation. For the interim analysis, 34 patients were screened, and 32 of them were treated.
Results: Seventeen male (53%) and 15 (47%) female patients, median age 69 (28-94) were treated. All patients received at least one prior line of rituximab-containing therapy mostly RCHOP (n=28, 87%). Ten (31%) participants received the IBR therapy as a second line and 22 (69%) as a third-line regimen, 6 (19%) received prior ASCT. Eight (25%) patients had relapsed disease, and 24 (75%) were refractory. Twenty-nine patients had denovo aggressive B cell lymphoma, including 12 (37%) ABC-DLBCL, 5 (16%) GCB-DLBCL, 6 (19%) undefined DLBCL, 2 (6%) primary mediastinal lymphoma and 4 (12%) double hit high grade lymphoma. Two patients had transformed low grade and 1 grade 3B follicular lymphoma.
We recorded 258 adverse events (AEs); 11 (4%) were Serious Adverse Events (SAEs) and 3 (1%) adverse events were of particular interest. SAEs that were related to ibrutinib were documented in 4 patients one time each (1.6%) and included atrial flutter/fibrillation, fatigue, and thrombopenia.
Of the 32 patients 27 were evaluable for response, the additional 5 patients had early clinical progression. Complete response (CR) was documented in 8 (30%), partial response in 4 (15%) and stable disease in 2 (7%) translating to overall response rate (ORR) of 45%. On an intention to treat analysis ORR was 38%. There were no statistically significant differences in response rates between relapsed vs. refractory patients as well as ABC type vs. others.
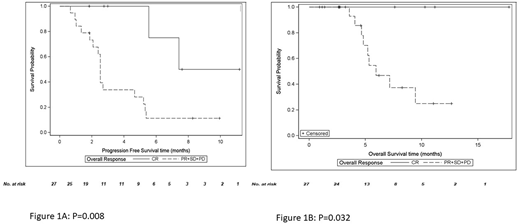
Four patients were referred to transplant after achieving CR or PR, 2 to ASCT and 2 to allogeneic stem cell transplantation. With a median follow-up of 14 months, all 4 patients remained alive and in remission. Median PFS and OS for the entire cohort (n=32) was 2.7 and 7.1 months, respectively. Both PFS and OS were significantly better for patients who achieved CR. Median PFS for patients with no CR was 2.5 months and was significantly different from patients with CR (p=0.008, log-rank test, figure 1a). Median OS for patients without a CR was 5.9 months; no deaths occurred among patients who achieved CR (p=0.032, log-rank test, figure 1b).
There were no significant differences in OS or PFS between relapsed and refractory patients as well as between the different histological subtypes.
Conclusions: We demonstrated that the IBR regimen is both safe and effective for patients with RR ABCL. Our patient population was of advanced age and mostly refractory, and despite that, we were able to show 45% ORR. Four of the patients that were chemorefractory and therefore transplant ineligible were able to undergo transplants after achieving remissions with this regimen, all of them are alive and in remission. We conclude that this regimen has promising results as a salvage regimen for RR ABCL patients both as a sole therapy or as a bridge to transplant, and it should be investigated in further clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal